Context
Swine Influenza A virus (swIAV) may transmit through aerosols. The virus has been detected in air samples collected in affected pig farms [1] and a relationship between airborne swIAV detection and the number of infected pigs was shown [2]. Here, we report swIAV detection in air samples collected in experimental rooms housing specific-pathogen-free (SPF) pigs without or with maternally-derived antibodies (MDA). Thirty-three MDA- piglets were assigned to 3 independent rooms (rooms 1 to 3) and 33 MDA+ piglets to 3 others (rooms 4 to 6) [3]. In each room there were 2 seeder pigs (intra-tracheally inoculated with A/Sw/France/Cotes d’Armor/0388/09 (H1N1) (106 EID50 in 5 mL) and 4 pen-mates in direct-contact, as well as 5 indirect-contact pigs in a neighboring pen, 30 cm apart. (Figure 1).
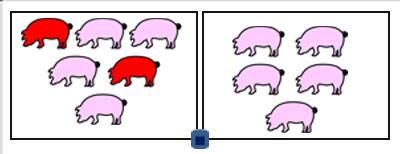
Figure 1: An experimental room was composed of two pens. The air sampler collector was placed in-between. The inoculated pigs are colored in red.
[1] Corzo, C. A. Culhane, M. Dee, S. Morrison, R. B. Torremorell, M. (2013). Airborne detection and quantification of swine influenza a virus in air samples collected inside, outside and downwind from swine barns. PLoS One, 8, e71444.
[2] Corzo, C. A. Romagosa, A. Dee, S. A. Gramer, M. R. Morrison, R. B. Torremorell, M. (2013). Relationship between airborne detection of influenza A virus and the number of infected pigs. The Veterinary Journal, 196 (171-175).
[3] Cador, C. Hervé, S. Andraud, M. Gorin, S. Paboeuf, F. Barbier, N. Quéguiner, S. Deblanc, C. Simon, G. Rose, N. (2016). Maternally-derived antibodies do not prevent transmission of swine influenza A virus between pigs. Veterinary Research 47:86.
Materials
Protocol
The Coriolis® µ was put down in the room between the 2 pens, 70 cm away from the ground, at the height of piglets but without direct contact with them (Figure 1).
Air samples were taken 3 times a week for 25 days post-infection (DPI). At each collection time, the collector ran during 10 min to collect 3000 L of aerosols in 15 mL of 0.005% Triton solution.
The air samples were then concentrated thanks to an ultrafiltration step using Amicon® Filter Device and centrifugation for 30 min at 3900 × g. Viral RNA was purified from 150 µL eluate and 5 µL RNA extract were tested by real-time M gene RT-PCR to detect the swIAV genome.
Nasal swabs were taken on a daily basis until DPI 14, then every 2 days. RNA was extracted from 200 µL supernatants and 5 µL submitted to RT-PCR.
Results
The swIAV genome was detected in aerosols from all rooms, from DPI 2 or DPI 4, until the end of the experiment (Figure 2). In each room the viral genome load peaked at DPI 9.
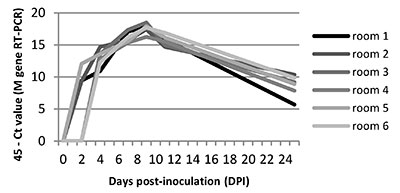
Figure 2. SwIAV genome load in air samples deduced from RT-PCR analyses (45-Ct value)
All indirect-contact (IC) pigs were shown to have been infected. They shed the virus from DPI 4 or DPI 6 depending on their serological status, and until DPI 14 for the last one (Table 1).
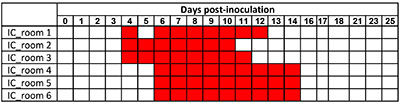
Table 1. SwIAV genome detection in nasal secretions of IC pigs. The room was considered positive (in red) when at least 1 out the 5 IC pigs was found RT-PCR positive.
All indirect-contact (IC) pigs were shown to have been infected. They shed the virus from DPI 4 or DPI 6 depending on their serological status, and until DPI 14 for the last one (Table 1).

